Professor.
BSc Honours (1987), PhD (1991) University of Cape Town. Robert A Welch postdoctoral fellow, Texas Christian University (1993). Lecturer, University of Cape Town (1994 - 1998), Senior lecturer (1998 - 2002), Associate Professor (2003 - 2007). Professor (2008 - present)
Email: susan.bourne@uct.ac.za
ORCID ID: https://orcid.org/0000-0002-2491-2843
Google Scholar: https://scholar.google.com/citations?hl=en&user=OlFQMW4AAAAJ
Research Interests
Chemists have developed the ability to tune the structural and electronic properties of many molecular species, which is useful in that it allows the design of new materials for a range of different applications including catalysis and optoelectronic effects. These applications require that molecules be aligned in the solid state so that the electronic and magnetic structures combine in the appropriate fashion. Control over solid state structures of molecular compounds is not straightforward, but a number of strategies have proved successful in promoting the adoption of predictable structures by molecular species. Thus, adding hydrogen-bonding groups to a molecule may lead to the formation of a predictable hydrogen-bonding network. Coordination polymerisation has also proved useful in aligning inorganic species . The encapsulation of a molecule into a crystalline host compound to form an inclusion compound may also produce ordered materials.
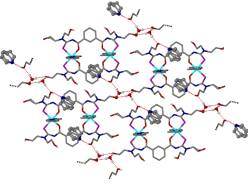
The concept of crystal engineering has provided a useful framework to develop rational approaches to solid-state structural design based on the self-assembly of molecular components. This field uses the concepts first developed in supramolecular chemistry; in particular recognizing the importance of hydrogen bonding, p¼p interactions and other “weak” interactions in generating and stabilizing larger networks. Of particular interest for their diverse functional properties are the “metal organic frameworks” or MOFs (porous polymeric materials, consisting of metal ions linked by organic bridging ligands)
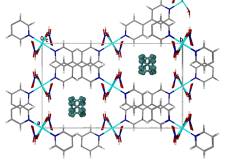
and layered hybrid perovskites (in which layers of metal centres are separated by organic layers with no covalent bonds linking the layers).
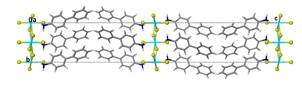
These materials are of interest because of their structural, magnetic, optical and electrical properties, particularly since these can be easily modified by replacement of the metal, halide or amine. The tendency of the hybrids to self-assemble from the solid or liquid phase arises from the range of interactions seen in these compounds, from van der Waals forces between organic components, hydrogen bonding within the organic layer or between organic and inorganic components to ionic and covalent bonds within the metal halide sheets.
We have a fruitful collaboration with Prof Klaus Koch (University of Stellenbosch) on the self-assembly of metallomacrocyclic complexes. Using thiourea derived ligands to form metallomacrocyclic complexes of platinum(II) or nickel(II), whose sizes (2:2 or 3:3 metal:ligand) depend only on the relative substitution (para vs meta) of the carbylthiourea moieties linked through a phenylene ring, we have explored the crystal engineering and inclusion properties of such metallomacrocycles. The Ni(II) example is interesting as it is readily converted to octahedral geometry through the coordination of two axial ligands perpendicular to the macrocycle plane.