A new vaccine hope for African horse sickness, from an unlikely source
Researchers at the University of Cape Town’s Biopharming Research Institute (BRU) have created a promising new vaccine candidate to help prevent the devastating effects of African Horse Sickness (AHS). And they’re producing it in tobacco plants.

The need for an effective AHS vaccine is pressing. The disease is a devastating one, particularly in Africa, with up to 90% of infected horses dying in some outbreaks. The current commercial vaccine is known as a live-attenuated vaccine, and while it remains effective, it carries some risks. According to Prof Alan Guthrie, Director of the Equine Research Centre at the University of Pretoria and a former collaborator on this project, live vaccines can and occasionally do cause outbreaks of their own.
“There are two problems with a live-attenuated virus vaccine - reassortment of the genome and reversion to virulence,” he says. “Both can lead to outbreaks, which is what happened in the Cape in three different AHS outbreaks over the last 15 years - in 2004, 2011, and 2014.”
This is why other parts of the world don’t use the currently-available vaccine, says Guthrie. And this is a looming threat, as a changing climate allows the midge that carries the virus to spread to new parts of Europe and the United Kingdom.
According to Sue Dennis, PhD candidate and lead author on this study, the BRU’s plantproduced vaccine doesn’t carry any of these risks, which makes it suitable for use around the world.
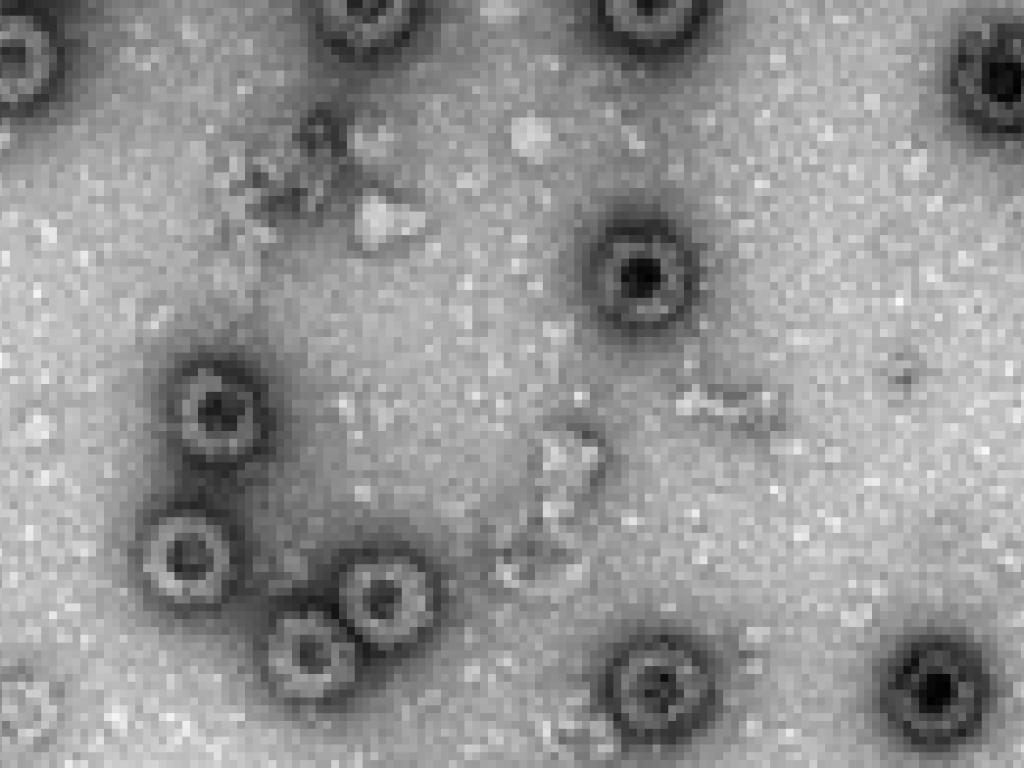
“We’ve used tobacco plants to produce four different virus proteins that automatically assemble to form a virus-like particle (VLP). It looks the same as the virus, just without any genetic material; so it cannot replicate or infect horses with the disease.
This VLP is the vaccine - when injected into an animal, the immune system produces antibodies to the virus that will fend off the real thing and protect the animal from disease. Dennis says that initial results look very promising, but there is more work to be done.
“When we tested the plant-produced vaccine in healthy horses, we saw an immune response at the same level as the live vaccine,” she says.When first testing vaccines in live animals, the most important thing is to show that the animal’s health is not affected, and that the immune system produces neutralising antibodies - the best indication that the vaccine will work against the live virus. On both counts, the BRU study has been a success.
“The presence of neutralising antibodies is a strong indication that horses will be protected from the virus,” she says. “But to confirm that the vaccine offers complete protection, we need what’s called a live challenge.”
In addition, the VLPs produced by Dennis and colleagues represent just one strain of AHSV; they are currently working on producing vaccines against the other strains. This success builds on more than 10 years of work at the BRU producing VLPs and other proteins in tobacco plants. In particular, years of work on bluetongue virus, which is related to AHS virus, has contributed to this breakthrough.
The next step is to test the protective power of the vaccine in horses against a challenge with live, virulent AHSV (the so-called live challenge), to see whether this promising vaccine candidate can stand up against the live virus. If it does as well as the current live-attenuated vaccine, BRU researchers believe they will be well on their way to a new global AHS vaccine.
This research was funded in part by the Technology Innovation Agency, and related intellectual property has been protected through UCT’s Department of Research, Contracts and Innovation, who receive a rebate from the DST National IP Management Office (NIPMO) to support patenting.
About BRU
The Biopharming Research Unit (BRU, Department of Molecular and Cell Biology at UCT) makes recombinant proteins in plants for use as diagnostics or vaccines for human and animal diseases. The Unit comprises research groups led by Professor Ed Rybicki, Associate Professor Inga Hitzeroth and Dr Ann Meyers, and boasts the largest portfolio of biotechnology patents at UCT, as well as the largest molecular biotechnology portfolio in South Africa. http://www.mcb.uct.ac.za/mcb/BRU-home
For media enquiries, please contact Dr Ann Meyers on 021 650 5712 | ann.meyers @uct.ac.za. To read the full paper, go to https://veterinaryresearch.biomedcentral.com/articles/10.1186/s13567-018-0600-4.
Press release written and distributed for the Biopharming Research Unit by ScienceLink.
