Prof Roger Hunter
7.33, PD Hahn building, upper campus
Biography
- Associate Professor (1989—2003) and Mally Chair (2004—present), University of Cape Town, RSA
- US Fulbright Fellow (2007), Yale University, USA
- Lecturer (1983-1988), University of the Witwatersrand, RSA
- EPSRC Post-Doctoral Research Fellow (1980-1982), University College, London, UK
- Royal Society European Science Exchange Post-Doctoral Fellow (1978—1980), Instituto Quimico de Sarria, Barcelona, Spain
- BSc Hons (1975), PhD, DIC (1978), Imperial College, London, UK
Research Interests
My interests centre on the application of synthetic organic chemistry to a broad range of problems in methodological, medicinal and total synthesis areas, as well as asymmetric catalysis.
1) Total Synthesis
A number of bioactive alkaloids (Castanospermine, Tacamonine, Lepadiformine) are being pursued via total synthesis, with the emphasis on new synthetic methodology.1—3 Recently, a new ephedrine–based auxiliary–controlled methodology has been developed for accessing a key chiral, non–racemic azaquaternary synthon relevant to Lepadiformine synthesis, as shown below:
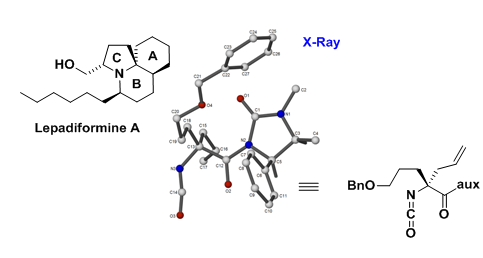
Organocatalysis is also being used to access azaquaternary centres, in which a novel hydrazide catalysis is being developed:

2) Medicinal Chemistry Projects
There are three major areas:
a) Novel Bifunctional HIV-1 Reverse-Transcriptase Inhibitors 4—6
The objective is to design, synthesize and develop HIV reverse-transcriptase bifunctional inhibitors containing a NNRTI and a NRTI separated by a tether so as to bind to each target site (a pocket and the polymerisation substrate-binding site respectively) simultaneously on RT. The project is in collaboration with Professor Karen Anderson at Yale University, USA. An example2 is shown below:
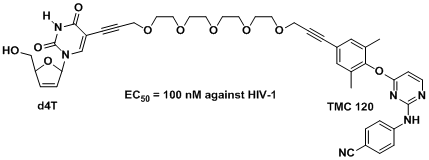
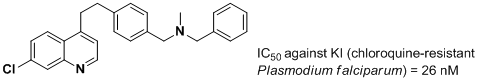
b) New Malaria Antimalarials (with Professor Tim Egan) 7,8
New chloroquine-based antimalarial hybrids incorporating a dibemethin grouping have been shown to be active against KI, the chloroquine–resistant strain of Plasmodium falciparum responsible for Malaria infection. An example is shown below:
c) Garlic mimics (with Dr Catherine Kaschula and Professor Iqbal Parker of the IDM) 9—11
Compounds resembling allicin and ajoene from garlic are being synthesized and developed as stable mimics with potent antimicrobial and anti–cancer properties. A structure–activity profile has been developed for the anti–cancer agent ajoene, shown below:
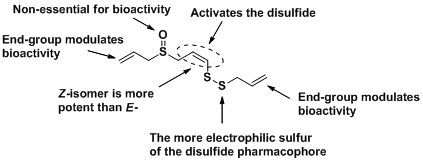
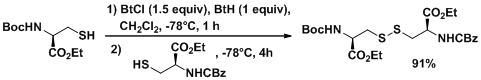
New methodology for unsymmetrical disulphide synthesis has been developed 12—14
Representative Publications
- Vinylogous Mukaiyama aldol reactions with 4-oxy-2-trimethylsilyloxypyrroles: relevance to castanospermine synthesis. Hunter, R,; Rees-Jones, S. C. M.; Su. H. Beilstein Journal of Organic Chemistry, 2007, 3, 38-43.
- 4,5-Erythro/5,6-threo-Stereoselectivity in vinylogous Mukaiyama aldol addition of a silyloxypyrrole to a threose derivative: stereochemical rationalization and relevance to (+)-castanospermine synthesis. Hunter, R.; Rees-Jones,, S. C. M.; Su, H. Tetrahedron Lett., 2007, 48, 2819-2822.
- A new approach to indolo[2,3-a]quinolizidines through radical cyclization of 2-acyl-1-phenylthiotetrahydro-b-carbolines bearing pendent a ,b -unsaturated esters. Smith, M. W.; Hunter, R.; Patten, D.; Hinz, W. Tetrahedron Lett., 2009, 50, 6342-6346.
- Current Developments in the Synthesis and Biological Activity of HIV-1 Double-Drug Inhibitors (Review). Hunter, R.; Muhanji, C.I. Current Medicinal Chemistry, 2007, 14, 1207-1220.
- C-2-Aryl O-substituted HI-236 derivatives as non-nucleoside HIV-1 reverse-transcriptase inhibitors. Hunter, R.; Younis, Y.; Muhanji, C. I.; Curtin, T-L.; Naidoo, K. J.; Petersen, M.; Bailey, C. M.; Basavapathruni, A.; Anderson, K. S. Bioorg. Med. Chem., 2008, 16, 10270-10280.
- [d4U]-spacer-[HI-236] double-drug inhibitors of HIV-1 reverse-transcriptase. Younis, Y.; Hunter, R.; Muhanji, C. I.; Hale, I.; Singh, R.; Bailey, C. M.; Sullivan, T. S.; Anderson, K. S. Bioorg. Med. Chem., 2010, 18, 4661â4673.
- A series of structurally simple chloroquine chemosensitizing dibemethin derivatives that inhibit chloroquine transport by PfCRT. Zishiri, V. K.; Hunter, R.; Smith, P. J.; Taylor, D.; Summers, R.; Kirk, K.; Martin, R. E.;. Egan, T. J. Eur. J. Med. Chem., 2011, 46, 1729-1742.
- Quinoline Antimalarials Containing a Dibemethin Group Are Active against Chloroquinone-Resistant Plasmodium falciparum and Inhibit Chloroquine Transport via the P. falciparum Chloroquine-Resistance Transporter (PfCRT). Zishiri, V. K.; Joshi, M. C.; Hunter, R.; Chibale, K.; Smith, P. J.; Summers, R. L.; Martin, R. E.; Egan, T. J. J. Med. Chem., 2011, 54, 6956-6968.
- Substituted ajoenes as novel anti-cancer agents. Hunter, R.; Kaschula, C. H.; Parker, I. M.; Caira, M. R.; Richards, P.; Travis, S.; Taute, F.; Qwebani, T. Bioorg. Med. Chem. Lett., 2008, 18, 5277-5279.
- Anti-proliferative activity of synthetic ajoene analogues on cancer cell-lines. Kaschula, C. H.; Hunter, R.; Hassan, H. T.; Stellenboom, N.; Cotton, J.; Zhai, X. Q.; Parker, M. I. Anti-Cancer Agents in Medicinal Chemistry, 2011, 11, 260-266.
- Structure-activity studies on the anti-proliferation activity of ajoene analogues in WHCO1 oesophageal cancer cells. Kaschula, C. H.; Hunter, R.; Stellenboom, N.; Caira, M. R.; Winks, S.; Ogunleye, T.; Richards, P.; Cotton, J.; Kilbeyaz, K.; Wang, Y.; Siyo, V.; Ngarande,, E.; Parker, M. I. Eur. J. Med. Chem., 2012, 50, 236-254.
- Inexpensive, One-Pot Synthesis of Unsymmetrical Disulfides using 1-Chlorobenzotriazole. Hunter, R.; Caira, M. R.; Stellenboom. N. J. Org. Chem., 2006, 71, 8268-8271.
- One-pot synthesis of unsymmetrical disulfides using 1-chlorobenzotriazole as oxidant: Interception of the sulfenyl chloride intermediate. Stellenboom, N.; Hunter,R.; Caira, M. R. Tetrahedron, 2010, 66, 3228-3241.
- A high-yielding, one-pot preparation of unsymmetrical glycosyl disulfides using 1-chlorobenzotriazole as an in situ trapping / oxidizing agent. Stellenboom, N.; Hunter, R.; Caira, M. R.; Szilágyi, L. Tetrahedron Lett., 2010, 51, 5309–5312.
