Prof Mino Caira
6.17, PD Hahn building, upper campus
Biography
- Professor (2002—present), University of Cape Town, RSA
- Associate Professor (1988—2001), University of Cape Town, RSA
- Post-Doctoral Fellowship (1983), Texas Christian University, Fort Worth, Texas, USA
- Lecturer, Senior Lecturer, Associate Professor (1976—1988), University of Port Elizabeth, RSA
- BSc Honours (1970), MSc (1971), PhD (1975), University of Cape Town, RSA
Research Interests
Research focuses on the modes of molecular association found in crystalline polymorphs, pseudo–polymorphs (solvates), inclusion compounds and molecular complexes, with an emphasis on pharmacologically–active substances. These systems are of theoretical and practical importance. Due to the high costs involved in the long–term development of new therapeutic agents, there is increased activity world–wide in the alteration of the dissolution characteristics of existing drugs with the aim of rendering them more, or less, soluble, depending on the application. This can be achieved by converting one polymorphic form of a drug into another, by preparing a solvated derivative or by complexing the drug with another molecule (e.g. a crown ether or a cyclodextrin). The modified species has a different crystal structure from that of the parent drug and the resultant difference in solubility and other physical properties can affect drug performance very significantly
Techniques used to identify and characterise new species prepared in this way include X–ray diffraction, thermal analysis and spectroscopic methods. The level of precision with which solid–state drug conformations and binding sites can be determined by single crystal X–ray analysis is unsurpassed by other physicochemical techniques. Furthermore, details of crystal packing modes revealed by this method assist in the interpretation of thermal degradation profiles and dissolution–rate data.
Drugs under current investigation include both new and well–established non–steroidal anti–inflammatories, antimicrobials, antimalarials and steroidal anticancer agents. Because of its applied nature, there is strong interest in this research from pharmaceutical companies. Several projects are undertaken in collaboration with research pharmacists at local and overseas institutions. The methodology employed in the study of these materials is the same as that used to investigate general host–guest systems, facilitating also my active involvement in this department’s wider programme on the chemistry of inclusion compounds.
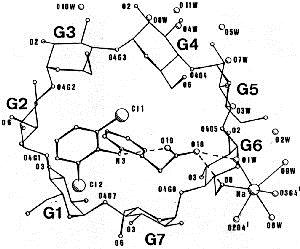
Inclusion of the anti–inflammatory drug Diclofenac in the cavity of a β–cyclodextrin molecule, revealed by single crystal X–ray diffraction.
Representative Publications
- Crystallization: Polymorphism. M R Caira. Encyclopedia of Separation Science. Wilson ID, Adlard ER, Cooke M and Poole CF (Eds.), Vol.3:975-985, 2000. London : Academic Press.
- On the isostructurality of cyclodextrin inclusion complexes and its practical utility. M R Caira. Rev. Roum. Chim., 2001, 46, 371-386.
- Thermal and structural properties of Ambroxol polymorphs. M R Caira, A Foppoli, M E Sangalli , L Zema and F Giordano. J. Therm. Anal. Cal., 2004, 77, 653-662.
- New crystalline forms of permethylated β–cyclodextrin. M R Caira, S A Bourne, P M Dean and W T Mhlongo. Chem. Commun., 2004, 2216-2217.
- Preparation, thermal behaviour and solid–state structures of inclusion complexes of permethylated β–cyclodextrin with the garlic–derived antithrombotics (E)– and (Z)–Ajoene. M R Caira, R Hunter, S A Bourne and V J Smith. Supramol. Chem., 2004, 16, 395-403.
- Isostructurality of Inclusion Compounds. M R Caira. Encyclopedia of Supramolecular Chemistry. Atwood, JL, Steed JW (Eds.), 767-775, 2004. New York : Marcel Dekker Inc.
- Relationships between structural and thermal properties of anhydrous and solvated crystalline forms of Brodimoprim. M R Caira, G Bettinetti, M Sorrenti and L Catenacci. J Pharm Sci., 2007, 96, 996-1007.
- Sulfa Drugs as Model Cocrystal Formers. M R Caira. Mol. Pharmaceutics, 2007 4, 310-316.
- Effect of peracetylation on the conformation of γ–cyclodextrin. M R Caira, G Bettinetti, M Sorrenti, L Catenacci, D Cruickshank and K Davies. Chem. Commun., 2007, 1221-1223.