Dr Gerhard Venter
7.09, PD Hahn building, upper campus
Biography
- BSc Hons (2000), MSc (2002), PhD (2005), University of Stellenbosch, RSA
- Postdoctoral fellow (2006—2007), University of Cape Town, RSA
- Lecturer (2007—2016), University of Cape Town, RSA
- Senior Lecturer (2017), University of Cape Town, RSA
Research Interests
The most obvious definition of an ionic liquid is a substance in the liquid state, consisting of positive cations and negative anions. Left unmodified, this definition would include simple ionic mixtures such as liquid NaCl as well, as long as it is applied above its melting point of >800°C. Room temperature ionic liquids (RTILs), on the other hand, are ionic mixtures with melting points (an arbitrarily chosen limit!) of <100°C, making them suitable for more moderate conditions. Popular RTILs consist of organic cations such as alkyl substituted imidazoliums, pyridiniums, pyrrolidiniums or ammoniums combined with inorganic or organic cations such as the halides, tetrafluoroborate, hexafluorphosphate and triflate and bistriflimide.
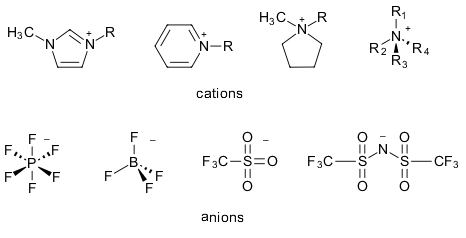
Depending on the chosen combination, physical properties such as melting point, viscosity and conductivity vary significantly. Within a family, further changes such as the length of the alkyl chain or functionalisation with e.g. ether groups, can also lead to a host of varying properties.
With a liquidus range of several hundreds of degrees (often the upper temperature limit is not governed by vaporization, but by decomposition) and a wide range of physical properties, the RTILs have numerous exciting applications. With the promise of being able to engineer an ionic liquid that suits the requirements of a specific application, the RTILs make ideal candidates to replace traditional molecular solvents in organic reactions, metal catalysis and electrochemistry.
Simulation and Calculation of RTIL Properties
Whichever application is focused on, a fundamental requirement common to all is a thorough understanding of the nature of the physicochemical properties of the RTILs. The idea of compiling structure-property relationships is intriguing, but rests on a solid understanding and prediction of the intermolecular interactions and related dynamic behaviour of these systems, and it is here where microscopic insight is needed that molecular simulation and calculation becomes a powerful tool. Quantum mechanical (QM) calculations can be used to investigate the electronic stucture of ions and ion pairs. Correctly chosen methods provide accurate descriptions of ion-ion interaction, polarization, hydrogen bonding and dispersion interaction. Molecular dynamics (MD) simulations can be used to integrate over the time evolution of a system, subject to a complete (and accurate) formulation of the intermolecular forces, which can subsequently provide further thermodynamic information and transport properties.
Developing structure-property relationships for room temperature ionic liquids require computational methods (static and polarizable force fields) that accurately describe the range of intermolecular interactions present. Developing such computational methods is one of the aims of this research. Furthermore, important insight into structure-property relationships can be gained through a better understanding of the nature of the interactions, and the effect of strengthening/weakening particular components of it.
Representative Publications
- D. A. Kusza, G. A. Venter, M. Mabunda, J. Biwi, S. K. Samanta, J. D. Klinck, S. V. Singh, R. Hunter and C. H. Kaschula. Finding the Ajoene Sweet-Spot: Structure-Activity Relations that Govern its Blood Stability and Cancer Cytotoxicity. ChemMedChem, 2024, 19, e202400087.
- T. A. Shmool, L. K. Martin, L. Bui-Le, I. Moya-Ramirez, P. Kotidis, R. P. Matthews, G. A. Venter, C. Kontoravdi, K. M. Polizzi and J. P. Hallett. An experimental approach probing the conformational transitions and energy landscape of antibodies: a glimmer of hope for reviving lost therapeutic candidates using ionic liquid. Chem. Sci., 2021, 12, 9528-9545.
- E. Batisai, G. A. Venter and N. B. Báthori. Formation of nitrobenzene dimers in racemic and chiral salts of 2-amino-1-(4-nitrophenyl)-1,3-propanediol (ANPD) with oxalic and fumaric acids. J. Mol. Struct., 2021, 1224, 129310.
- M. W. Smith, J. Ferreira, R. Hunter, G. A. Venter and H. Su. Synthesis of (+)-Tacamonine via Stereoselective Radical Cyclization. Org. Lett., 2019, 21, 8740-8745.
- K. N. Sharara, K. Nyamayaro, M. M. Wicht, G. A. Venter and N. B. Báthori. Multicomponent crystals of nitrofurazone–when more is less. CrystEngComm, 2019, 21, 1091-1096.
- N. Joondan, S. B. Moosun, P. Caumul, S. N. Sunassee, G. A. Venter and S. Jhaumeer-Laulloo. Effect of chain length on the interactions of sodium N-alkyl prolinates with bovine serum albumin: a spectroscopic investigation and molecular docking simulations. Colloid Polym. Sci., 2018, 296, 367-378.
- L. Mongalo, A. S. Lopis and G. A. Venter. Molecular dynamics simulations of the structural properties and electrical conductivities of CaO–MgO–Al2O3–SiO2 melts. J. Non-Cryst. Solids, 2016, 452, 194.
- D. Kuter, V. Streltsov, N. Davydova, G. A. Venter, K. J. Naidoo and T. J. Egan. Solution structures of chloroquine–ferriheme complexes modeled using MD simulation and investigated by EXAFS spectroscopy. J. Inorg. Biochem., 2016, 154, 114.
- D. Kuter, V. Streltsov, N. Davydova, G. A. Venter, K. J. Naidoo and T. J. Egan. Molecular Structures and Solvation of Free Monomeric and Dimeric Ferriheme in Aqueous Solution: Insights from Molecular Dynamics Simulations and Extended X-ray Absorption Fine Structure Spectroscopy. Inorg. Chem., 2014, 53, 10811.
- B. W. Weber, S. W. Kimani, A. Varsani, D. A. Cowan, R. Hunter, G. A. Venter, J. C. Gumbart and B. T. Sewell. The Mechanism of the Amidases: Mutating the Glutamate Adjacent to the Catalytic Triad Inactivates the Enzyme due to Substrate Mispositioning. J. Biol. Chem., 2013, 288, 28514.
