Assoc Prof Anwar Jardine
7.12, PD Hahn building, upper campus
Biography
- PhD (1995), University of Cape Town, RSA
- Andrew Mellon Foundation Fellowship (1996—1997), University of Cape Town Medical School, RSA
- Harvard Medical School/South African Medical Research Council Fellowship (1998—1999)
Research Interests
Medicinal Chemistry, Chemical Biology, Enzymology, Natural product a, Organic synthesis, Drug Design and Development
Research Summary
The inseparable nature of Chemistry and Biology has encouraged many productive collaborations leading to the establishment of this relatively new research and teaching environment. The chemistry and biology of poly-, oligo- and monosaccharides and their enzymatic processing is particularly interesting in health and disease. Our focus is on the applied chemistry and value addition of abundant natural marine polymers, such as chitin and alginates. Our interest also includes targeting small molecule carbohydrate biomarkers that has been proven critical for the survival of pathogenic bacteria.
Research Description
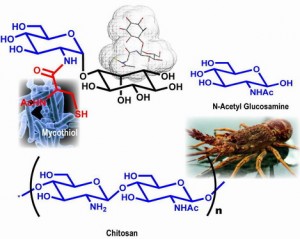
Mycothiol processing enzymes as potential anti-mycobacterial drug targets: All organisms growing in an aerobic environment produce one or more low molecular mass thiol(s) as a major metabolite. In the majority of eukaryotes, gluthathione (GSH) is the principal antioxidant thiol in gram negative bacteria. In prokaryotes and certain eukaryotes, gluthathione (GSH), trypanothione (TSH) and thioredoxin (Trx), maintains the cellular redox homeostasis. Many gram positive bacteria, such as M.tb, lack glutathione and produce a number of alternate thiols. Most Actinomycetes produce mycothiol (MSH) as their principal low molecular mass thiol (1D-myo-inosityl-2-(N-acetyl-L-cysteinyl)-amido-2-deoxy-alpha-D-glucopyranoside). Present knowledge indicates that the redox cycling of mycothiol (MSH) to mycothiol disulfide (MSSM) is very critical for the in vivo and in vitro survival of mycobacteria. Since MSH is only present in Actinomycetes and plays an important protective role, all the enzymes involved in its maintenance are potential drug targets. A wealth of knowledge exists for the structure and function of glutathione reductase and trypanothione reductase, but the analogous activity of mycothiol disulfide reductase has not yet been thoroughly investigated. In the absence of a crystal structure of mycothiol disulfide reductase, we are interested in developing potential lead inhibitors of the mycothiol pathway enzymes.
Chitin and Chitosan: Chitin is the second most abundant natural biopolymer in the world, behind only cellulose.It is a heterogeneous polysaccharide that consists of beta-1,4-linked N-acetylglucosamine residues that are arranged in antiparallel (alpha), parallel (beta), or mixed (gamma) strands, with the alpha configuration being the most abundant. It is also the most abundant naturally occurring polysaccharide that contains amino sugars. This abundance, combined with the specific chemistry of chitin and its N-deacetylated derivative chitosan, make for the array of potential applications. Chitin and chitosan have already found applications in diverse products that have reached the market. Chitin occurs as a component of crustacean shells, insect exoskeletons, fungal cell walls, microfauna, and plankton, it is found in association with proteins and minerals such as calcium carbonate. Further breakdown of chitosan yields N-acetylglucosamine and glucosamine. These amino sugars have therapeutic potential for the treatment of a variety of diseases, including arthritis, inflammatory bowel disease, and general inflammatory damage
In nature, degradation of all forms of chitin is carried out efficiently by the combined activity of the enzymatic systems of a multitude of microorganisms. The pilot scale study of chemical, enzymatic or chemoenzymatic methods for the production of chitosan is under investigation. Our research focus involves further value addition by the synthesis and evaluation of modified chitosan for general commercial and biomedical applications.
Representative Publications
- Claus Bornemann, Anwar M. Jardine, Daan J. Steenkamp and HendrikS.C.Spies. The Biosynthesis of Mycothiol: Elucidation of the sequence of steps in Mycobacterium Smegmatis. Biochem. J., 1997, 325, 623-629.
- Jardine, M. Anwar; Spies, Hendrik S. C.; Nkambule, Comfort M.; Gammon, David W.; Steenkamp, Daniel J. Synthesis of mycothiol, 1D-1-O-(2-[N-acetyl-L-cysteinyl]amino-2-deoxy-alpha-D-glucopyranosyl)-myo-inositol, principal low molecular mass thiol in the actinomycetes. Bioorg. Med. Chem. (2002), 10, 875-881.
- Leonidas, Demetres D.; Chavali, Gayatri B.; Jardine, Anwar M.; Li, Songlin; Shapiro, Robert; Acharya, K. Ravi. Binding of phosphate and pyrophosphate ions at the active site of human angiogenin as revealed by X-ray crystallography. Protein Sci. (2001), 10, 1669-1676.
- Jardine, Anwar M.; Leonidas, Demetres D.; Jenkins, Jeremy L.; Park, Chiwook; Raines, Ronald T.; Acharya, O.K. Ravi; Shapiro, Robert. Cleavage of 3′,5′-Pyrophosphate-Linked Dinucleotides by Ribonuclease A and Angiogenin. Biochemistry (2001), 40, 10262-10272.
- Kumar Kapil; Jenkins Jeremy L; Jardine Anwar M; Shapiro Robert. Inhibition of mammalian ribonucleases by endogenous adenosine dinucleotides. Biochem. Biophys. Research Commun. (2003), 300, 81-6.
- Book Chapter: Jardine, Anwar. Synthesis of naturally occuring isoflavones and their analogs. pp225-242, in Pueraria, The genus Pueraria, Medicinal and Aromatic Plants- Industrial Profiles, edited by Wing Ming Keung, Taylorand Francis, Londonand New York(2002) ISBN: 0-415-28492-9
